MEDICAL DEVICE HARDWARE AND SOFTWARE DEVELOPMENT
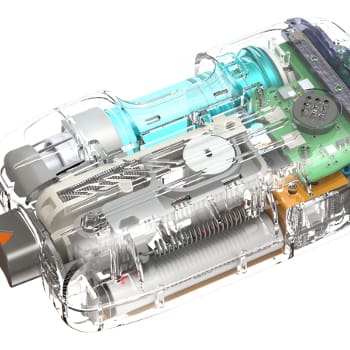
NOVO specializes in medical device development services for Class I, II, and III FDA-regulated devices. Our quality management system is certified to ISO 13485:2016, and incorporates our five-phase product development process (PDP). The scope of our PDP spans product definition through manufacturing transfer, including support for clinical trials and regulatory submissions. Our medical device engineering expertise encompasses both hardware development and medical device software development. For medical devices that have hardware, embedded software (firmware), and application software components, an engineering partner that offers all three can be a significant advantage.
Medical device development differs from commercial hardware and software development in the level of human risk and regulatory oversight. There is a formal requirement to demonstrate safety and efficacy. Mistakes in planning or execution during medical device development projects will have a greater impact on time to market and total investment. A partnership with an experienced engineering team, familiar with the regulatory environment and compliant with all relevant standards, offers greater predictability for startups and established medical device companies alike.
MEDICAL DEVICE DEVELOPMENT PROJECTS WITH NOVO
NOVO’s PDP is designed to steadily reduce technical risk and build confidence in the design approach with each incremental milestone. Thoughtful planning allows parallel development activities that compress the schedule without adding additional development risk. Frequent hardware and software integrations allow system-level testing and characterization of critical parameters. Materials and manufacturing processes are considered from the beginning of the development. Design control documentation is generated concurrent with the development for straightforward integration into the design history file. Compliance and future regulatory filing are an ongoing consideration throughout the project.
At the outset of a new medical device project, NOVO’s senior-level medical device engineering team works with clients to identify key technical risks and resolve them. With the technology well characterized, system architecture and design input requirements can be confirmed. At this point, a complete project plan that addresses partner roles and responsibilities, design and development, risk management, verification testing, regulatory submissions, clinical trials, and manufacturing strategies can be drafted.
With the foundation for an organized and efficient development now laid, the design and development process can begin in earnest. The value of NOVO’s onsite development tools, product development processes, and specialized engineering staff becomes evident during this period, evidenced by requirements verified and milestones met.
A prototype assembly line at NOVO for building design verification and compliance testing devices also provides an effective method of transferring design intent knowledge to the contract manufacturing partner. Once the design is transferred, NOVO provides ongoing support for design validation activities including clinical trials and regulatory filings.
At all stages of development, our objective is to increase our client’s confidence that foreseeable issues have been addressed, and to demonstrate that the criteria for moving to the next phase of development have been met.
General Medical Device Development Services
- ISO 13485-certified
- Development of verifiable design input requirements
- Concept development
- Functional, marketing, and clinical trial prototypes
- Risk and hazard analysis and FMECA
- Device detail design and development
- Preclinical trial management
- Biocompatibility and toxicity test management
- Sterilization validation
- Device verification testing and software validation testing
- Industrial design (ID), human factors (HF), and user interface (UI) design
- Design control and DHF documentation
- Compliance with ISO, IEC, FDA QSR
- Process validation and automated test equipment (ATE) qualification
Specific Medical Device Experience
NOVO’s medical device development team has experience with the following therapeutic and surgical device types:
- Endoscopy/laparoscopy
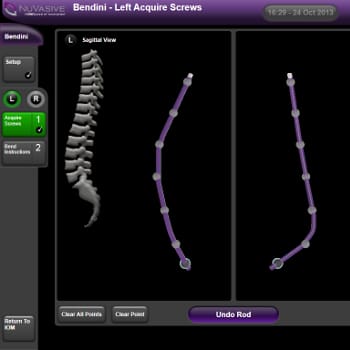
- Spinal implant surgery software
- RFID tagging and detection
- Wearable drug delivery devices
- Diabetes therapy devices
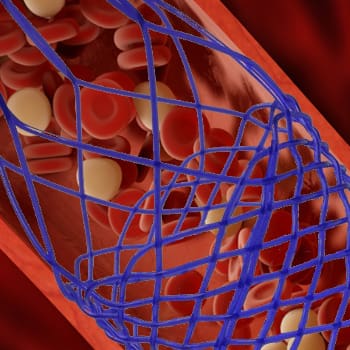
- Stent delivery systems
- Autoinjectors and pen injectors
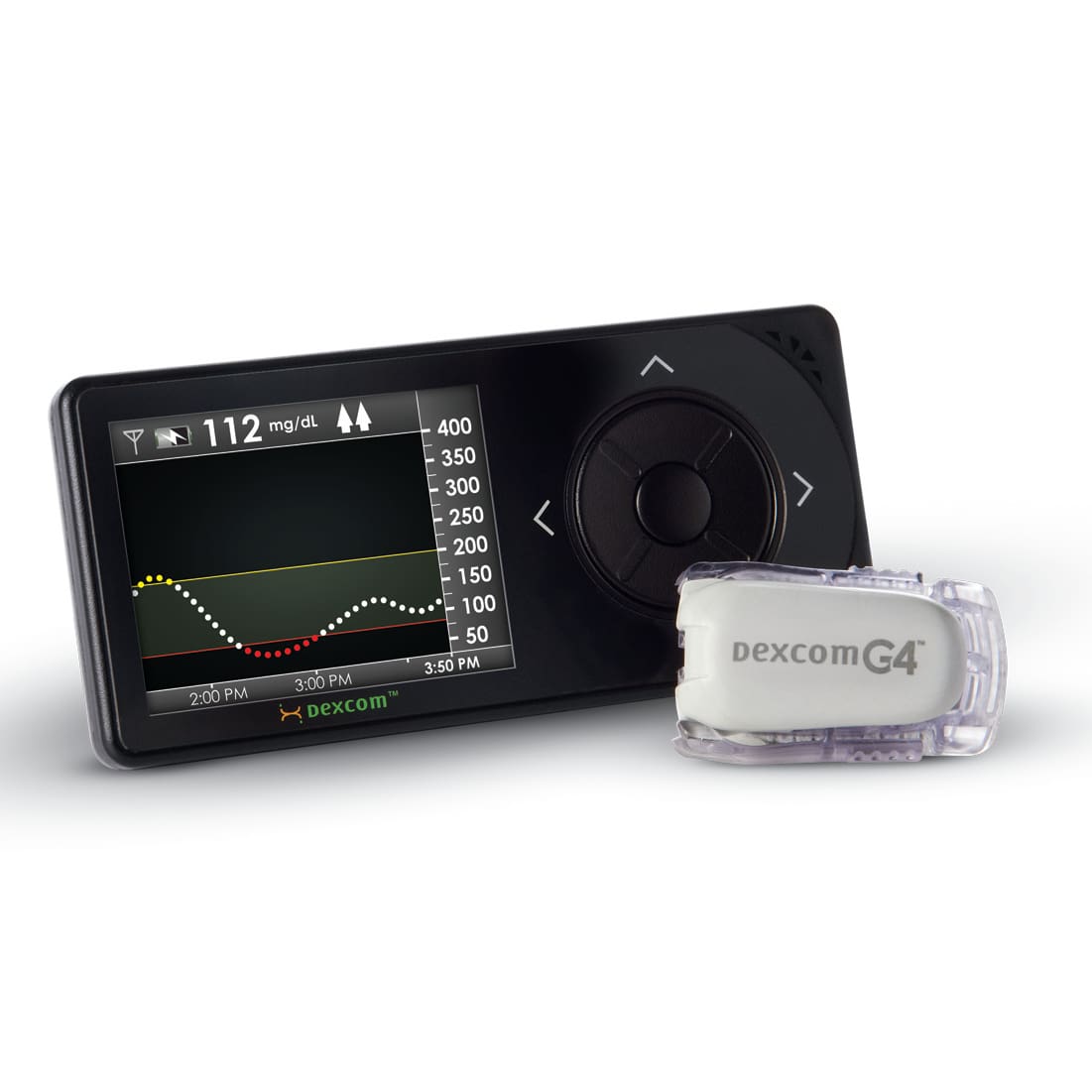
- Continuous glucose monitoring (CGM) systems
- Biofeedback devices
- Electroporation Devices
- Software as a medical device (SaMD)
- Surgical instruments and devices
- Proprietary device controllers
- Durable wireless transmitters
- Mobile device controllers
- Insertion devices and applicators
- Transcatheter aortic valve replacement (TAVR)
- Balloon catheters
- Needle, cannula, and trocar assemblies
- Dental implants
- Orthopedic implants
- User interface development