THE UNITY DIABETES MANAGEMENT SYSTEM
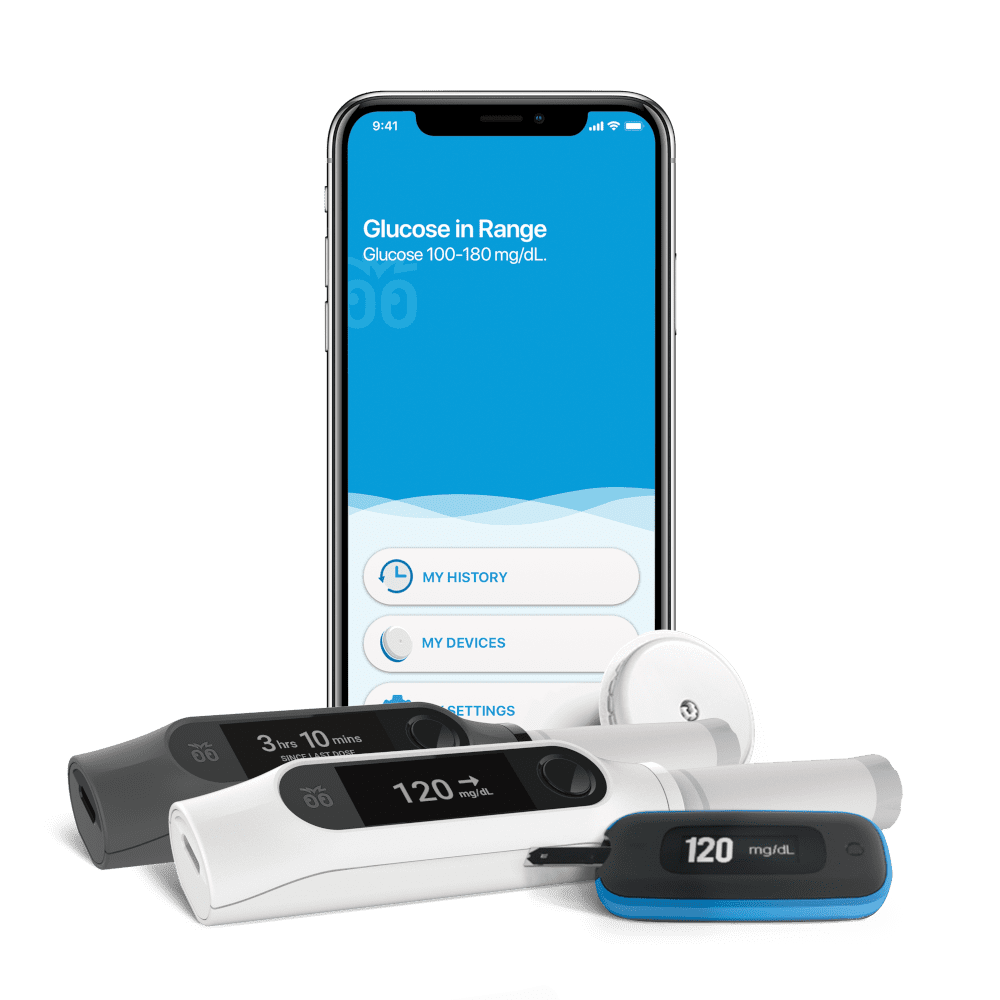
In mid 2021, Bigfoot Biomedical introduced the Unity™ Diabetes Management System after receiving FDA 510(k) clearance. The system features first-of-their-kind smart pen caps for insulin pens used to treat Type 1 and Type 2 diabetes. The Unity Diabetes Management System represents a major step forward in Bigfoot’s quest to reduce the cognitive burden of living with diabetes.
The smart pen caps offer compatibility with popular rapid and long acting disposable insulin pens; on-demand dose decision support, integration with Abbott’s FreeStyle Libre 2 integrated continuous glucose monitoring system (iCGM), automatic data sharing of CGM data with treating physician, integration with a blood glucose meter (BGM), and a mobile application for convenient system monitoring.
NOVO has been Bigfoot’s primary outside engineering partner since the company was founded. We are proud to have been a part of the development of this visionary product.
The Importance of This Product

To appreciate the value of the Unity smart pen cap system, imagine what a person with diabetes does on a daily basis to avoid serious health problems. They must monitor their glucose levels, keep track of what they eat, have a detailed understanding of their metabolism, maintain an accurate record of the amount and type of insulin they inject, and carefully plan their activities. A system that relieves the stress of these activities can significantly improve quality of life.
As Bigfoot puts it, the Unity Diabetes Management System answers the question, “how much insulin would my doctor recommend I take right now?”
NOVO’s Role in the Development

On the Unity program, our product development support included system architecture, electromechanical design, user-interface implementation, firmware development, quality engineering, test automation, and manufacturing transfer.
Integration and co-development of third party devices into a larger, networked ecosystem was a significant part of the engineering challenge. This effort required the use of multiple wireless protocols and implementation of wireless communication security without sacrificing ease of use.
Security was a design consideration throughout the development process, from sourcing through to recycling. A chain of trust was established from the device to the cloud. Securing medical device wireless communications protects the user from malicious interference by hackers, and the company from reputation risk.
Early in the development program, NOVO developed custom automated test equipment (ATE) to track and measure all key performance criteria. Systematic use of these ATE platforms allows controlled iteration and refinement of the device. Additional advantages of investing in ATE early include faster cycles of innovation and reduced burden for verification of changes.
Connected Medical Device Expertise
Product development for connected medical devices is an area of specialization for NOVO. Our hardware, software, and systems engineers thrive on tough projects like the Unity Diabetes Management System. We have 60+ engineers across the primary engineering disciplines, and an extensive product development infrastructure to support rapid product development for complex medical systems.
Patents Associated With This Project
The following is a partial list of patents covering different aspects of the Unity system that name NOVO engineers as inventors:








