DIAGNOSTIC PLATFORM EXPERTISE:
- Instrument design
- Consumables design
- Detection
- Imaging
- Fluidics
- Surface modification
- Sensing
- Thermal control
- Precision motion control
- Precision dispensing
- Mixing
EXPERTISE CONTINUED…
- Fluorescent immuno-assays
- Lateral flow assays
- Custom cartridge design
- Test card development
- Sample preparation
- Workflow automation
- Custom electronics development
- Micro molding
- User interface design
- IOT/connectivity
DIAGNOSTIC DEVICE DEVELOPMENT EXPERIENCE
NOVO is steadily building our expertise in diagnostic device development by taking on system and sub-system level projects for both screening and diagnostic applications. Typically, our clients perform the basic science and demonstrate initial feasibility before contacting us to help them develop a commercial implementation of the instrument and consumables. Because of NOVO’s expertise in the technologies required to commercialize diagnostic systems, our clients rely on us for hardware, software, and systems engineering development. Together, we transform the processes they have demonstrated on the lab bench into world-class point-of-care and laboratory diagnostic testing platforms.
SCREENING DEVICES
Screening devices are used to perform screening tests that detect potential disease indicators or risk factors early in the disease process. They are typically faster, less invasive, or less expensive than diagnostic tests. A positive result signals the clinician to perform diagnostic tests that definitively establish the presence or absence of a specific disease.
NOVO was a primary engineering partner in the development of Veriskin’s TruScore melanoma screening device — recently awarded FDA Breakthrough Device Status — which implements their proprietary dynamic epidermal capillary measurement (DECAM) technology. This technology uses non-invasive, optical measurement of pressure-induced cutaneous hemodynamics to detect pathologies in malignant lesions. The TruScore device is a significant step forward in the early detection of melanoma, which is a critical step in preventing the disease from spreading throughout the body and improving treatment outcomes.
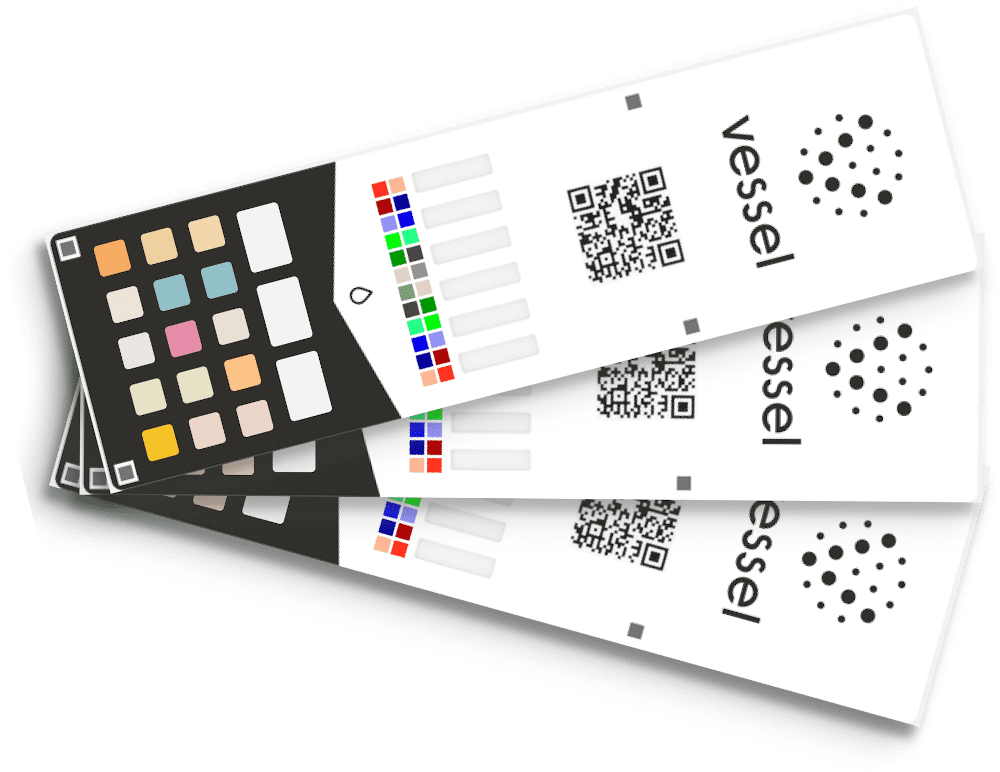
NOVO provided engineering support to Vessel Health in the development of their wellness test card that uses lateral flow and colorimetric technology to measure more than ten biomarkers in urine that are related to wellness. Biomarkers tested for include vitamins B7, B9, and C; Cortisol, Magnesium, and Ketones A and B; Creatinine, Calcium, hydration, and pH. Our contributions included test card development including a calibration method for mobile phone cameras to read the test card.
Low cost consumables development for diagnostic and screening devices is a natural extension of the fluid handling and high-volume manufacturing process knowledge we have acquired from our inkjet printing and biotech instrument work. Consumables development is key to the success of nearly every diagnostic platform and requires a broad understanding of materials, processes, fluidics, tooling, and manufacturing automation.
DIAGNOSTIC INSTRUMENTS AND DEVICES
NOVO’s subsystem-level responsibility for diagnostic devices, cartridges, and consumables includes design updates to existing fluorescent immunoassay (FIA) and lateral flow assay (LFA) platforms and ongoing development for next-generation platforms. We are experienced with the development of durable instruments (readers, analyzers) and with the development of cartridges and consumables. We can provide resources to integrate into your existing development team or provide turnkey hardware and software development services for diagnostic device platforms.
INSTRUMENTS AND READERS
NOVO has contributed to development and sustaining engineering for immunoassay instruments — analyzers, readers, meters. We have provided hardware and software development support for optical subsystems, image processing, automated library preparation, system architecture for next-gen PCR sequencing, a POC blood testing platform, automated cartridge and chip loading mechanisms, custom thermal cyclers, user interfaces, sensing systems, and enclosures. The durable consumable model is part of our DNA at NOVO. We have deep experience in making appropriate tradeoffs between the technology built into the single-use consumables and the technology that resides in the durable device.
A key benefit that NOVO can offer our clients is rapid and predictable development under our ISO-certified quality management system and product development process. Under this system, the documentation that will be incorporated into regulatory submissions is generated in parallel with product development activities. Our development processes are complemented by strong project management, a gifted engineering team, and a facility extensively equipped with prototyping and testing equipment. In combination, these attributes enable NOVO to deliver fully functional, integrated systems for testing within months of project start in many cases.
CARTRIDGES, CASSETTES, AND CONSUMABLES
Our work on cartridge, cassette, and test card development for diagnostic platforms includes early-stage cartridge design and development, optimization of sample port geometries, PCR chambers, reconstitution chambers, extraction chambers and mixing chambers, metering, anti-splashing, electronic control of fluid flow, a low-cost, bead-based PCR cartridge, and temperature measurement of cassettes.
Additional consumables development work includes reagent and buffer reservoir designs and quality control automation for detecting coating uniformity defects on consumables.
ARE YOU READY TO TALK?
We hope you are persuaded that NOVO is a good choice as a development partner for diagnostic startups and established diagnostic device companies that have R&D outsourcing needs. We’re standing by to answer any questions you might have. If you are ready to talk about advancing your diagnostic technology into a commercial product, then please contact us to set up an appointment.