Developing a Fixture and Method to Test Pushability and Trackability
Client Need & Project Overview
A leading medical device company needed a clinically relevant method to evaluate whether its flagship stent system met ISO requirements for deliverability. Specifically, the client sought to measure “pushability” and “trackability,” two critical parameters defined in ISO 25539-2:2012 (“Cardiovascular implants—Endovascular devices—Part 2: Vascular stents”).
NOVO developed a custom in-vitro test model and method to replicate real-world conditions of stent delivery. This enabled the client to generate reliable, reproducible data to support regulatory submission and product development.
Today, the medtech industry is rapidly adopting simulation, in-silico testing, and predictive modeling to accelerate validation, reduce reliance on animal testing, and lower development costs. While these approaches have become mainstream more recently, NOVO’s work on this project foreshadowed the shift toward simulation-driven device evaluation.
Key Engineering Challenges & Solutions
| Challenge | NOVO’s Solution |
|---|---|
| Replicating coronary anatomy | Designed test plates simulating the left coronary artery tree at multiple levels of tortuosity, including the aortic arch. |
| Clinical realism | Built a model to accommodate standard catheterization lab accessories (guide catheters, guidewires); immersed it in 37°C water to simulate body conditions. |
| Capturing device interactions | Developed a test procedure that replicated clinical maneuvers, including guide catheter back-out. If the guide catheter became dislodged, the attempt was ended. |
| Quantitative and qualitative metrics | Conducted a fixed number of delivery attempts at three levels of guide catheter support, collecting numerical tracking distances and operator ratings for pushability (force transfer efficiency) and trackability (smoothness through tortuosity). |
| Flexibility for future testing | Designed interchangeable test plate inserts parametrically, allowing new configurations for line extensions while maintaining baseline anatomical fidelity. |
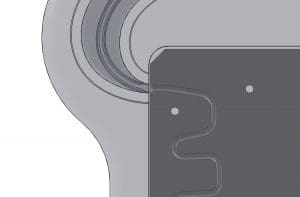
Technical Innovations & System Design
Fixture & Model Development
Engineered a reusable test fixture with modular inserts to mimic coronary anatomy under varying levels of tortuosity.
Simulated Clinical Environment
Integrated water immersion at body temperature, along with guidewire and catheter use, to replicate real procedural conditions.
Data Collection Methodology
Captured both quantitative tracking distance and qualitative operator feedback to provide a comprehensive picture of device deliverability.
Results & Impact
- The method and model supported CE mark submission by providing clinically relevant and reproducible data.
- Testing showed that the device under evaluation outperformed its predicate system in both pushability and trackability.
- The parametric design of the test plates allowed the model to be quickly adapted for product line extensions, reducing engineering time for future evaluations.
Looking back, NOVO’s approach anticipated today’s emphasis on simulation-driven validation. The same principles of patient-specific modeling, virtual testing, and predictive analytics are now reshaping medical device development. NOVO’s early focus on clinically realistic benchtop models positioned its clients to benefit from practices that would later define industry best standards.
Why NOVO Engineering?
NOVO combines deep engineering expertise with a systems-level perspective to help clients validate complex devices under clinically relevant conditions. By bridging simulation, usability, and regulatory needs, we deliver solutions that accelerate development while aligning with emerging industry trends.
Read about stent conformability testing related to this project here.
Want to explore how benchtop and simulation models can reduce risk and accelerate your medical device program?
Contact NOVO today to discuss your next innovation.