INSULIN PUMP DEVELOPMENT EXPERIENCE FOR PATCH STYLE AND TRADITIONAL PUMPS
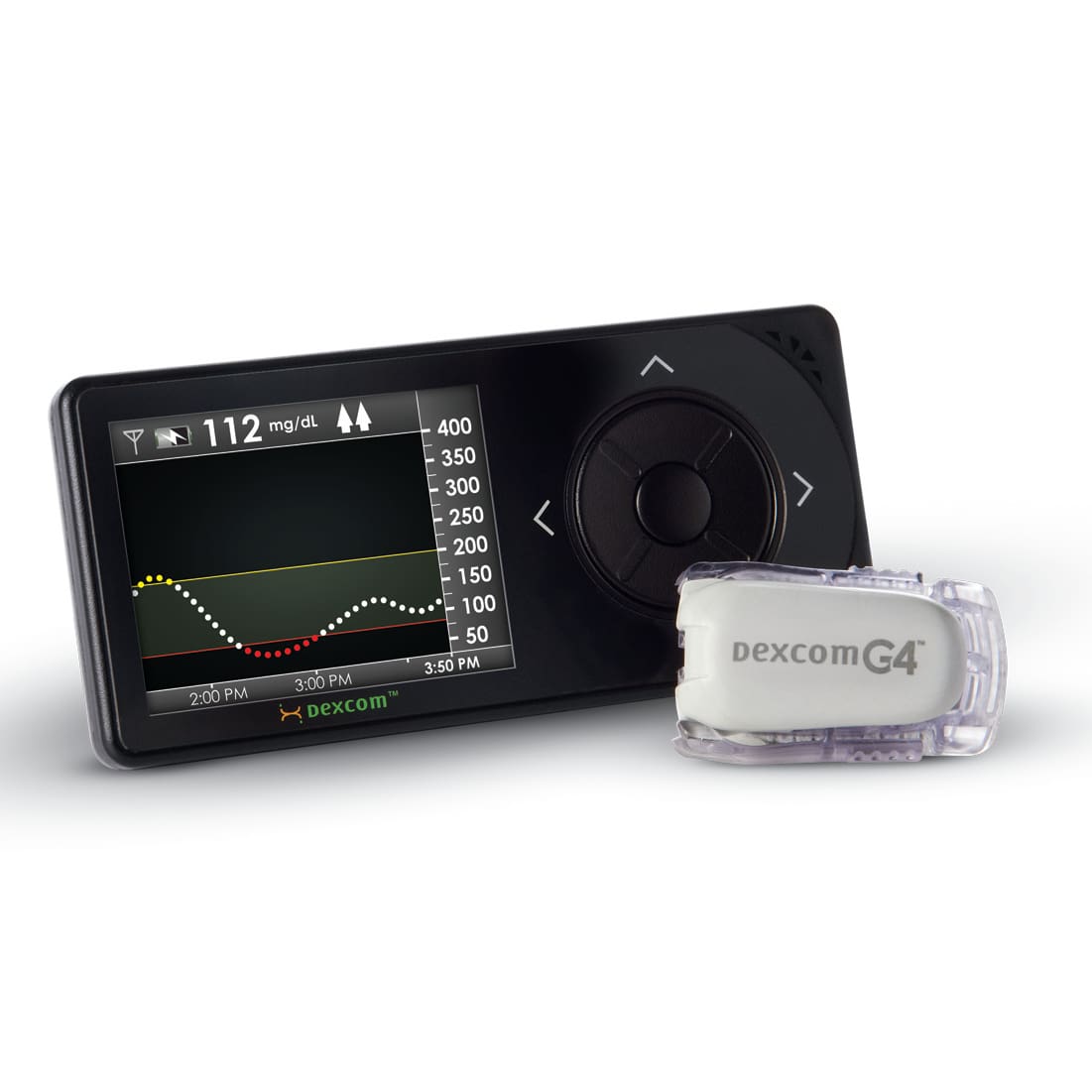
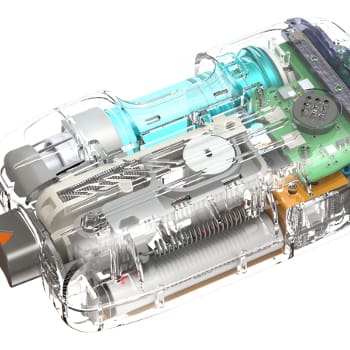
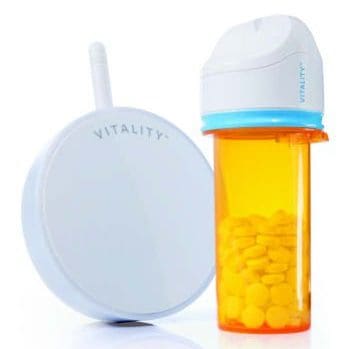
NOVO has contributed to the mechanical, electrical, and software design of several insulin pump product families including pumps, controllers, consumables, and accessories. The image above shows the Medtronic 670G (a component of the first FDA approved artificial pancreas system) , the Tandem Diabetes t:slim, and the Unilife Imperium pumps. NOVO contributed to each of these designs, and to several others still under development. Our experience extends to every subsystem in either a belt-worn or wearable pump (patch pump), including core systems like the pump drive, automatic cannula insertion/retraction, primary container, occlusion sensing, electronics, and embedded software.
In addition to product development, NOVO has developed automated test equipment and process control automation for insulin pump manufacturing and quality control purposes.
NOVO has integrated peripheral devices such as wireless continuous glucose monitors (CGM devices) and blood glucose meters, in addition to developing pump control electronics and Bluetooth low energy (BLE) communications. The breadth of our experience in diabetes care device development made NOVO an obvious choice as a development partner for an automated insulin delivery system development program currently underway. Our strong understanding of the design and manufacturing of diabetes care devices for both hospital and ambulatory applications makes us a unique resource to product companies bringing new insulin pumps, pen-injectors, and CGM devices to market.
INSULIN PUMPS: TECHNICAL CHALLENGES
Insulin pumps present a unique combination of technical challenges related to usability, performance, and reliability requirements. For example, usability requirements drive a goal of minimizing the size of the device, which in turn complicates the achievement of performance and reliability goals. Miniature, precision drive trains, occlusion sensing, environmental hardening for shock and water ingress, electrostatic discharge (ESD) hardening, wireless communications, system integration, manufacturability, and most importantly, patient safety combine to increase the technical challenges in these designs. Regulatory requirements and manufacturing considerations for both high-volume consumables and lower-volume durable assemblies add further challenges to insulin pump development.
THE ENGINEERING BEHIND GREAT PRODUCTS
NOVO has a history of helping our clients solve the most challenging aspects of the durable and consumable designs on several insulin pump projects. We have worked closely with our clients’ operations departments to create automated test equipment and assembly automation. NOVO continues to build our expertise in this important medical device product category, and in the related category of on-body drug delivery devices, as we provide product, process, and manufacturing development support to our clients developing the next generation of diabetes care products.
Related case studies include: