Client Need & Project Overview
The rise of minimally invasive therapies for weight management has driven demand for smarter, more patient-friendly devices. Years before digital health solutions and connected care became mainstream, Obalon Therapeutics partnered with NOVO Engineering to advance intragastric balloon technology — enhancing inflation system design, pressure regulation, and control architecture.
Obalon, a leader in non-surgical weight loss treatments, needed engineering expertise to improve the performance of their gas-filled intragastric balloon system while preserving the established system footprint. NOVO supported multiple development initiatives, including balloon and valve design, inflation system improvements, and system optimization, helping Obalon deliver a more streamlined, reliable, and scalable solution.
Key Engineering Challenges & Solutions
| Challenge | NOVO’s Solution |
|---|---|
| Pressure regulation in a compact system | Designed an optimized pressure manifold within severe space constraints, avoiding major changes to the PCBA, mechanical enclosures, or overall device size. |
| System reliability adhering to regulatory requirements | Conducted detailed power consumption analyses and implemented ESD solutions to meet IEC 60601-1-2 standards without disrupting manufacturing processes. |
| Software development within Obalon’s ecosystem | Set up the build environment, supported requirements documentation, developed and reviewed code, configured static analysis, and contributed to unit testing and design verification. |
| Accelerated development timeline | Delivered solutions compatible with existing architecture to minimize impact on validation timelines and support a timely FDA Pre-Market Approval (PMA) submission. |
Technical Innovations & System Design
Mechanical & Electrical Design
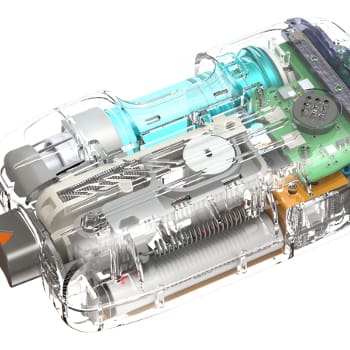
- Re-engineered the pressure manifold with minimal impact to surrounding components, optimizing air discharge and system reliability.
- Added sensors, electronically controlled pressure regulation, and closed loop control for pressure regulation during fill.
- Enhanced safety features.
- Integrated ESD mitigation strategies to meet stringent medical device EMC requirements.
- Ensured all modifications fit seamlessly within existing mechanical and electronic constraints.
Software & Controls Development
- Supported the establishment of robust software development processes.
- Created and refined embedded software aligned with Obalon’s quality management system (QMS).
- Performed static analysis, peer reviews, issue investigations, code revisions, and contributed to risk and protocol documentation.
Human Factors & Usability Enhancements
- Enabled more automated, controlled balloon inflation, simplifying clinical workflows.
- Reduced variability during deployment, improving both clinician confidence and patient outcomes.
Results & Impact
- Enhanced Device Performance – Successfully iterated the inflation system with improved control and ease of use, without requiring disruptive system redesign.
- Streamlined Clinical Workflow – Automation of the inflation process enhanced procedural efficiency and consistency for clinicians.
- Regulatory Progress – Supported Obalon’s efforts toward successful PMA submission and regulatory approvals.
- Early Alignment with Future Trends – Introduced automation and precision control that reflect standards now common in minimally invasive therapies.
Why NOVO Engineering?
Today’s healthcare environment demands precision, automation, and user-centric device designs. NOVO’s early work with Obalon showcased a vision for more sophisticated, digitally enhanced control in bariatric devices — years before it became a market expectation.
By combining expertise in precision control, embedded software, and human-centered system design, NOVO demonstrated how smart automation could enhance procedural consistency, reduce clinical variability, and improve patient outcomes. These advancements anticipated key industry trends that have since become essential to minimally invasive therapies. Today’s bariatric and endoscopic interventions increasingly rely on automation to deliver safer, more predictable results.
This forward-thinking approach continues to distinguish NOVO: solving complex engineering challenges today while helping clients align with the technologies and clinical needs of tomorrow.
Ready to Accelerate Your Medical Device Innovation?
Contact NOVO today to explore how we can help you overcome technical challenges and bring next-generation devices to life.