Developing an In-Vitro Method to Evaluate Stent Conformance and Safety
Client Need & Project Overview
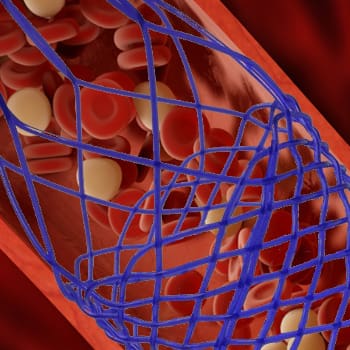
A medical device company sought to validate the conformability of a next-generation coronary artery stent. Conformability, or how well a stent conforms to the arterial wall, is critical to patient safety because poorly apposed stent segments can obstruct blood flow and increase the risk of thrombosis and acute myocardial infarction.
The client’s initial evaluation method relied on low-power visual inspection, which could not capture the interior of the stent or reliably identify feature defects. To ensure regulatory readiness and patient safety, the client needed a robust in-vitro benchtop method to objectively measure stent conformability.
Today, advanced imaging and benchtop simulation are driving the medtech industry toward more precise and non-invasive evaluation methods. Years before CT-based inspection became widely used for device characterization, NOVO was pioneering this approach to strengthen design verification.
Key Engineering Challenges & Solutions
| Challenge | NOVO’s Solution |
|---|---|
| Improve internal imaging | Developed a novel test method using an existing inspection instrument that enabled detailed visualization of stent interiors and feature detection. |
| Realistic anatomical modeling | Designed two test plates: one with straight channels and one with 90° bends of decreasing radii, sized for artificial arteries, stent thickness, and balloon compliance. |
| Accurate deployment conditions | Delivered stents into artificial arteries and expanded them to nominal and rated burst pressures, enabling realistic evaluation of conformance. |
| Quantification of conformability | Created a repeatable framework for inspecting stents post-deployment, allowing objective comparisons across product generations. |
| Inside and outside imaging | Introduced use of a CT scanner already on-site at the client facility, enabling virtual 3D inspection of stents in situ. |
Technical Innovations & System Design
Benchtop Test Plates
Custom-designed channels replicated straight and bent arterial paths, supporting both nominal and burst pressure testing.
Artificial Artery Integration
Incorporated artery wall thickness, stent dimensions, and balloon compliance into the model for accurate simulation.
Conformance Measurement
Stent system samples were delivered into artificial arteries and expanded to nominal and rated burst pressures, deploying the stent against the vessel wall. Upon pressure release, each stent retained a level of conformance to the artery relative to the bend and radius of the channel, allowing direct visual and quantitative comparison across designs.
CT-Based 3D Imaging
Repurposed a CT scanner for detailed visualization of both the inside and outside of deployed stents, providing novel insight into conformance.
Quantitative Quality Framework
Enabled the client’s quality engineering team to characterize defects and establish clinically relevant acceptance criteria.
Results & Impact
- The novel method significantly improved evaluation accuracy, going beyond traditional light inspection to capture detailed images of stent interiors and identify subtle variations in conformance.
- By using a CT scanner already available at the client’s facility, the approach proved highly cost-effective and eliminated the need for new, specialized imaging systems.
- The consistent test framework made it possible to benchmark performance across multiple stent generations, strengthening both design verification and product validation.
- The project helped the client’s quality engineering team establish acceptance criteria that were directly linked to patient safety and real-world performance outcomes.
NOVO’s approach, which introduced CT-based inspection years before it became a widely used tool in device development, anticipated today’s emphasis on advanced imaging and simulation-driven validation. These methods are now integral to ensuring the safety and performance of cardiovascular implants, underscoring the visionary nature of NOVO’s engineering contributions.
Why NOVO Engineering?
NOVO combines expertise in mechanical design, advanced imaging, and clinically relevant modeling to help clients evaluate complex devices. By pairing innovative test methods with practical use of available resources, NOVO delivers solutions that accelerate regulatory readiness and ensure patient safety.
Read about testing for stent deliverability conforming to ISO 25539-2:2012 here.
Looking to improve device testing with advanced in-vitro models and imaging techniques?
Contact NOVO today to explore how we can help bring your next innovation to life.