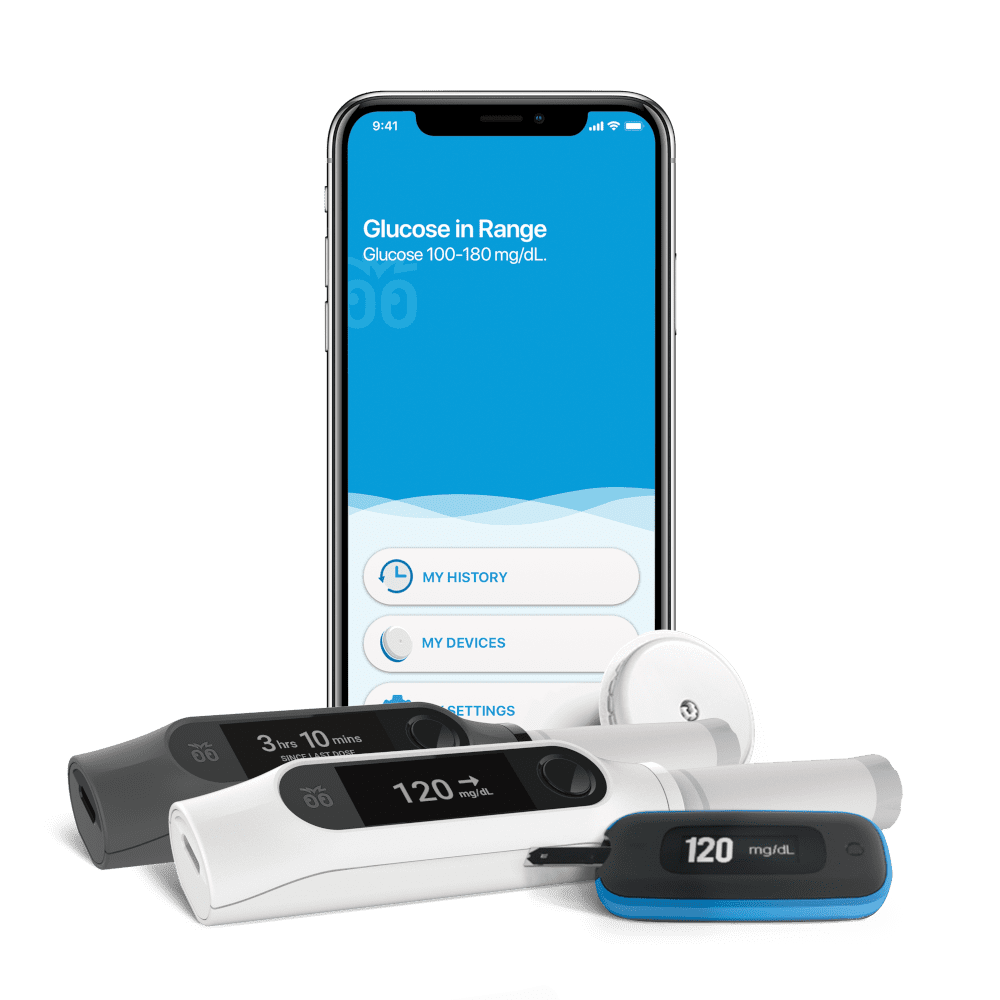
We congratulate our long-time client Bigfoot Biomedical on receiving FDA 510(k) clearance for their Unity™ Diabetes Management System. The system features first-of-their-kind smart pen caps for insulin pens used to treat Type 1 and Type 2 diabetes. The Unity Diabetes Management System represents a major step forward in Bigfoot’s quest to reduce the cognitive burden of living with diabetes.
The connected smart pen caps offer: Compatibility with popular rapid and long acting disposable insulin pens; on-demand dose decision support; integration with Abbott’s FreeStyle Libre 2 continuous glucose monitor (CGM); automatic data sharing of CGM data with treating physician; and a mobile application for convenient system monitoring.
NOVO has been Bigfoot’s primary outside engineering partner since the beginning. On the Unity program, our product development support included system architecture, electromechanical design, user-interface implementation, firmware development, and quality engineering, and manufacturing transfer.
Being associated with the launch of a product that has the potential to improve quality-of-life for people with diabetes adds another layer of satisfaction to a fun and challenging program. Great work Bigfoot Unity team!
Some of the other work that NOVO has done with our friends at Bigfoot includes hardware and firmware development for a smart insulin pump that will incorporate Bigfoot’s automated insulin delivery technology.
Also, if you are new to NOVO, we invite you to watch our newly-minted company introduction video below.