DEVELOPING A SWALLOWABLE INTRAGASTRIC BALLOON AND DELIVERY SYSTEM
THE CLIENT’S REQUEST
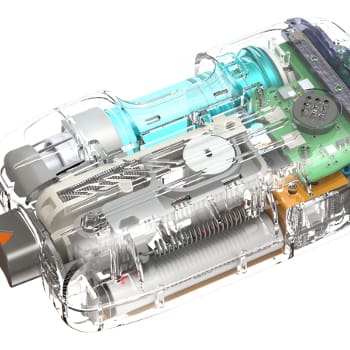
NOVO’s client, Obalon Therapeutics, was developing an intragastric balloon to help obese patients who have difficulty losing weight through dietary modification. The client asked NOVO to help with balloon and fill port design, development of the delivery mechanism, and development of the process for manufacturing the balloons, which were constructed from non-homogeneous technical films. The system consisted of an inflatable balloon contained within a gel capsule and tethered by an inflation tube. After ingestion, the balloon was inflated and the inflation tube withdrawn, leaving only the balloon in place to occupy volume in the stomach and regulate appetite. NOVO’s participation expanded to include the development of a method to disconnect the inflation tube from the balloon after implantation, a mechanical back-pressure regulator, an electronic pressure regulation system, embedded software, and user interface programming.

GASTRIC BALLOON: THE TECHNICAL AND DESIGN CHALLENGES
Technical challenges related to the balloon design included creating a robust interface between the inflation tube and the capsule containing the balloon, initiating the separation between the balloon and the inflation tube, and designing the detailed configuration of the balloon itself. Design of the seams, the heat-sealing process, thermoforming of technical laminated film, and the oragami-like method of folding the deflated balloon to fit within the gel cap, were all technical and design challenges. Chemical resistance to the acids in the stomach and physical robustness to abrasion and constrictions in the stomach were further challenges. The final geometry of the inflated balloon was critical, and all exposed seams and surfaces needed to be smooth and free from irregularities that might irritate the lining of the digestive tract.
The mechanical back-pressure regulator consisted of a precision-machined regulator assembly that would regulate an over-pressurized supply canister to an accurate set point pressure. It included an in-factory adjustment for precise calibration.
The electronic pressure regulation system had to perform the same functions as the mechanical system it replaced, in addition to compensation for ambient pressure variations due to altitude. Medical device electronics, firmware, and software design were part of the development. A graphical user interface was incorporated with animated physician instructions for use during the procedure. The device was battery operated, and as always, power management was a constant design consideration.
THE ENGINEERING BEHIND GREAT PRODUCTS
NOVO worked closely with client engineers through multiple configurations of the product design and manufacturing process. The delivery system, inflation control, valve, and balloon were bench-tested at NOVO, then transferred to the client’s manufacturing facility for clinical prototypes. A miniature valve assembly was designed and a method for joining the valve to the balloon developed. Forming and folding methods for the balloon itself were developed as well. A proprietary joint design for seams between layers of film was developed to minimize stresses that resulted from the joining operations. Equipment and methods for testing the device were developed in parallel with the device.
Clinical trial results on the device resulted in significant weight loss with no adverse effects. The system received premarket approval (PMA) from the FDA in record time, and Obalon is now a publicly traded company on the NASDAQ (OBLN). This project provides another example of how NOVO’s engineering expertise can be applied to help create products that have a significant impact on people’s quality of life.
NOVO’s work on this project has resulted in the following issued US patents to date: