EMBEDDED DESIGN FOR AN AUTOMATED INSULIN DELIVERY SYSTEM

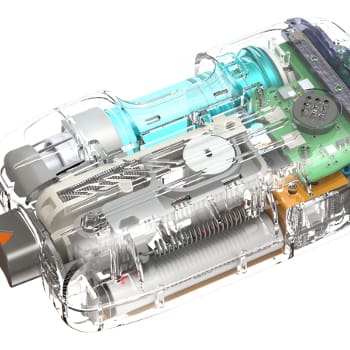
Type 1 diabetes (T1D) management is notoriously difficult and stressful for patients and their caregivers, who must monitor blood glucose, adhere to strict dietary requirements, and administer medication injections multiple times per day based on unique calculations and sometimes guesswork to determine the correct dose. The advent of insulin pumps, continuous glucose monitors (CGMs), and pen injectors have reduced the patient burden, but they are separate devices incapable of approximating the functions of a pancreas without significant interpretation and input by the patient. The founders of Bigfoot Biomedical recognized the opportunity to create an even greater improvement in the quality of life of T1D patients by integrating several (mostly) existing medical devices into an automated medication delivery system. Novo provided the insulin pump embedded design for this system, among other contributions.
Bigfoot chose NOVO to develop the missing component of the system: an insulin pump controller to coordinate the operation of the other devices in the network. NOVO provided the electronics hardware, embedded software (firmware), and enclosure design for the custom pump controller. NOVO also manufactured the devices used for Bigfoot’s first clinical trial under our ISO 13485:2016 certification.
The elements of the solution Bigfoot envisioned included a blood glucose meter (BGM), CGM, mobile medical application, web services (with cloud-based data analytics capabilities and portals for patients and providers), FDA-approved insulin pump body, and the custom insulin pump controller. This type of automated medication delivery system was formerly referred to by the FDA as an artificial pancreas system because it approximates the function of a human pancreas, which is to regulate blood glucose through the production of insulin. Because blood glucose levels that are significantly out of range can result in negative long-term health effects, including death, automated insulin delivery systems are designated as Class III medical devices by the FDA.
The controller NOVO designed uses multi-role capabilities supported by the Bluetooth 4.1 standard. It establishes the body-area network by communicating with each of the system components and manages medication delivery using patient-specific, proprietary algorithms developed by Bigfoot and implemented in the controller’s embedded software (firmware).
Firmware Solution #1: Secure Connected Health
The security of medical devices in general, and infusion pumps in particular, is of great concern to regulatory bodies and the InfoSec community. Although security beyond standard BLE encryption was not within NOVO’s original scope, NOVO engineers took the initiative to design for all data at rest to be encrypted, knowing that this would be required in the commercial device. When the product nears commercialization, the controller will be able to securely store medically sensitive patient data involved in diabetes management; furthermore, the software architecture will neatly accommodate encryption for all data in transit, which increases system security and protects patient privacy in a highly connected medical device.
Firmware Solution #2: Integrated Medical Device Software Test Framework
In a 2002 report, the US FDA CDRH stated that 79% of software-related medical device recalls were attributed to software defects introduced when software was changed after initial production and distribution.1 To address this risk, continuous integration testing was incorporated early in the software development plan. To accomplish this, a method of automatically testing the firmware without impacting software runtime in an appreciable way would be necessary.
The developers were able to create a data model that separated test framework communications from other internal communications, so tests can run without affecting the behavior of the system. The only meaningful impact was a small increase in RAM.
The Engineering Behind Great Products
NOVO’s insulin pump embedded design contributions to this medical device development program, including the incorporation of control algorithms developed by Bigfoot, integrated a set of discrete devices into a body area network that functions as a closed loop medication delivery system—a major step along the road to creating a true artificial pancreas. Clinical trials are now underway for this Class III automated insulin delivery device.
Learn about our electronics hardware and enclosure development for this product here.
Here is a video from Bigfoot.
Reference:
- Center for Devices and Radiological Health. (2002, January 11). General Principles of Software Validation; Final Guidance for Industry and FDA Staff (Document UCM085371). Retrieved September 1, 2016. Document access here.